Daily News | Online News
By The Korea Advanced Institute of Science and Technology (KAIST)
October 11, 2022
The researchers believe that their approach might be a breakthrough in treating Alzheimer’s disease without triggering inflammatory side effects or synapse loss.
The new drug circumvents the neurotoxic inflammatory side effects that occur with conventional treatments.
Aduhelm, a monoclonal antibody that targets amyloid beta (A), recently became the first United States Food and Drug Administration (FDA)-approved drug for
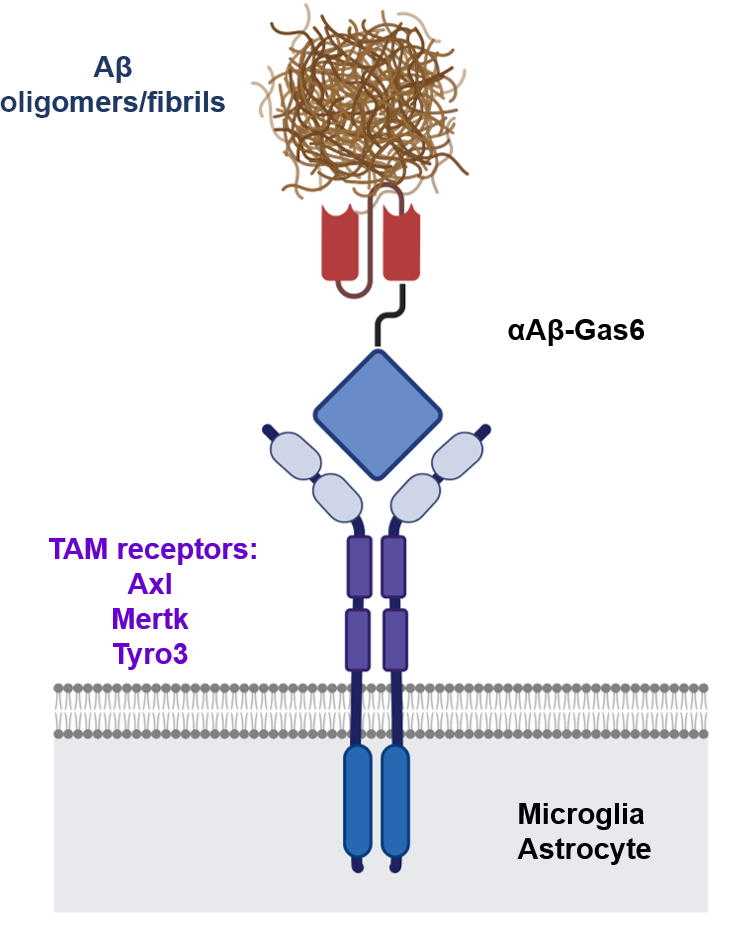
Schematic of a chimeric Gas6 fusion protein. A single chain variable fragment (scFv) of an Amyloid β (Aβ)-targeting monoclonal antibody is fused with a truncated receptor binding domain of Gas6, a bridging molecule for the clearance of dead cells via TAM (TYRO3, AXL, and MERTK) receptors, which are expressed by microglia and astrocytes. Credit: Gliabiology Lab & Kim Lab of Immunotherapy
These inflammatory side effects have the potential to worsen cognitive impairment in Alzheimer’s disease patients by causing neuronal cell death and synapse elimination by activated microglia. As a result of its inflammatory side effects, current Aβ antibody-based immunotherapy has the risk of inflicting more damage than good.
To address these issues, a team of researchers at the Korea Advanced Institute of Science and Technology (KAIST) in South Korea created Aβ-Gas6, a new fusion protein drug that successfully eliminates Aβ through a completely different mechanism than Aβ antibody-based immunotherapy. In a mouse model of Alzheimer’s disease, A-Gas6 not only eliminated Aβ more effectively, but it also avoided the neurotoxic inflammatory side effects associated with conventional antibody treatments.
The findings were recently published in the journal Nature Medicine.
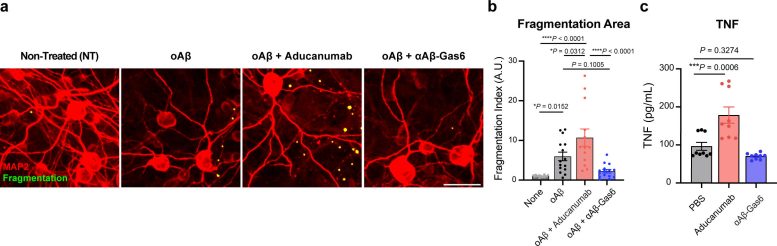
The resulting αAβ-Gas6 clears Aβ oligomers and fibrils without causing neurotoxicity (a-b, neurons: red, and fragmented axons: yellow) and proinflammatory responses (c, TNF release), which are conversely exacerbated by the treatment of an Aβ-targeting monoclonal antibody (Aducanumab). Credit: Gliabiology Lab & Kim Lab of Immunotherapy
“FcR activation by Aβ targeting antibodies induces microglia-mediated Aβ phagocytosis, but it also produces inflammatory signals, inevitably damaging brain tissues,” said paper authors Chan Hyuk Kim and Won-Suk Chung, associate professors in the Department of Biological Sciences at
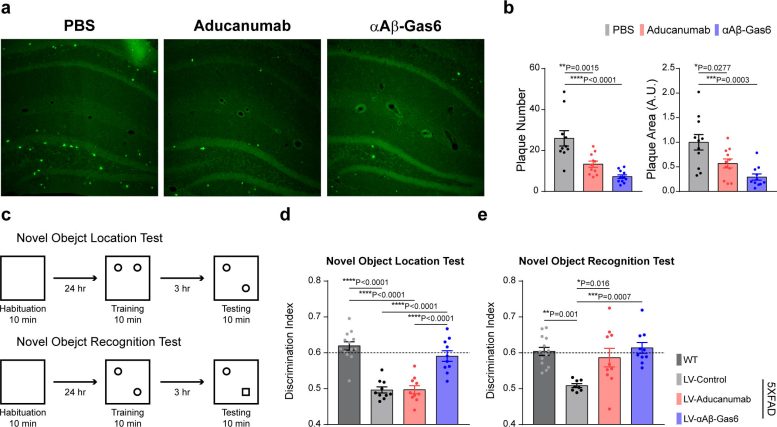
The number and total area of Aβ plaques (Thioflavin-T, green) were significantly reduced in αAβ-Gas6-treated AD mouse brains compared to Aducanumab-treated ones (a, b). The cognitive functions of AD model mice were significantly rescued by αAβ-Gas6 treatment, whereas Aducanumab-treated AD mice showed a partial rescue in these cognitive tests (c-e). Credit: Gliabiology Lab & Kim Lab of Immunotherapy

0 Comments :
Post a Comment